Actionable Information – Research Briefs – 1 -Vaccination
Last updated: March 24, 2021
Suggested citation:
Z-Losoya, E., Medina-Cetina, Z., Pompelli, G., Cochran, M., Olivares, M., Perez-Patron, M., Duran, G. & Alvarado, J. (2021). Actionable Information – Research Briefs – 1 – Vaccinations. https://r13-cbts-sgl.engr.tamu.edu/actionable-information-research-briefs-1-vaccination/
@misc{Z-Losoya2021,
author = {Z-Losoya, Enrique and Medina-Cetina, Zenon and Pompelli, Gregory and Cochran, Matthew and Olivares, Miriam and Perez-Patron, Maria Jose and Duran, Guillermo},
title = {Actionable Information - Research Briefs -1 - Vaccinations},
url={https://r13-cbts-sgl.engr.tamu.edu/actionable-information-research-briefs-1-vaccination/},
year={2021},
month={March}
}Table of Contents
- U.S. COVID-19 Vaccines Key Takeaways
- U.S. Vaccination Overview and Supply Chain
- Moderna’s Manufacturing Facilities and Supply Chain Overview
- Johnson and Johnson Janssen COVID-19 Vaccine
- COVID-19 Vaccination Landscape
- References
U.S. COVID-19 Vaccines Key Takeaways
- Pfizer, Moderna, and J&Js Janssen COVID-19 Vaccines have been granted emergency use authorization in the United States (see Table tbl. 1)
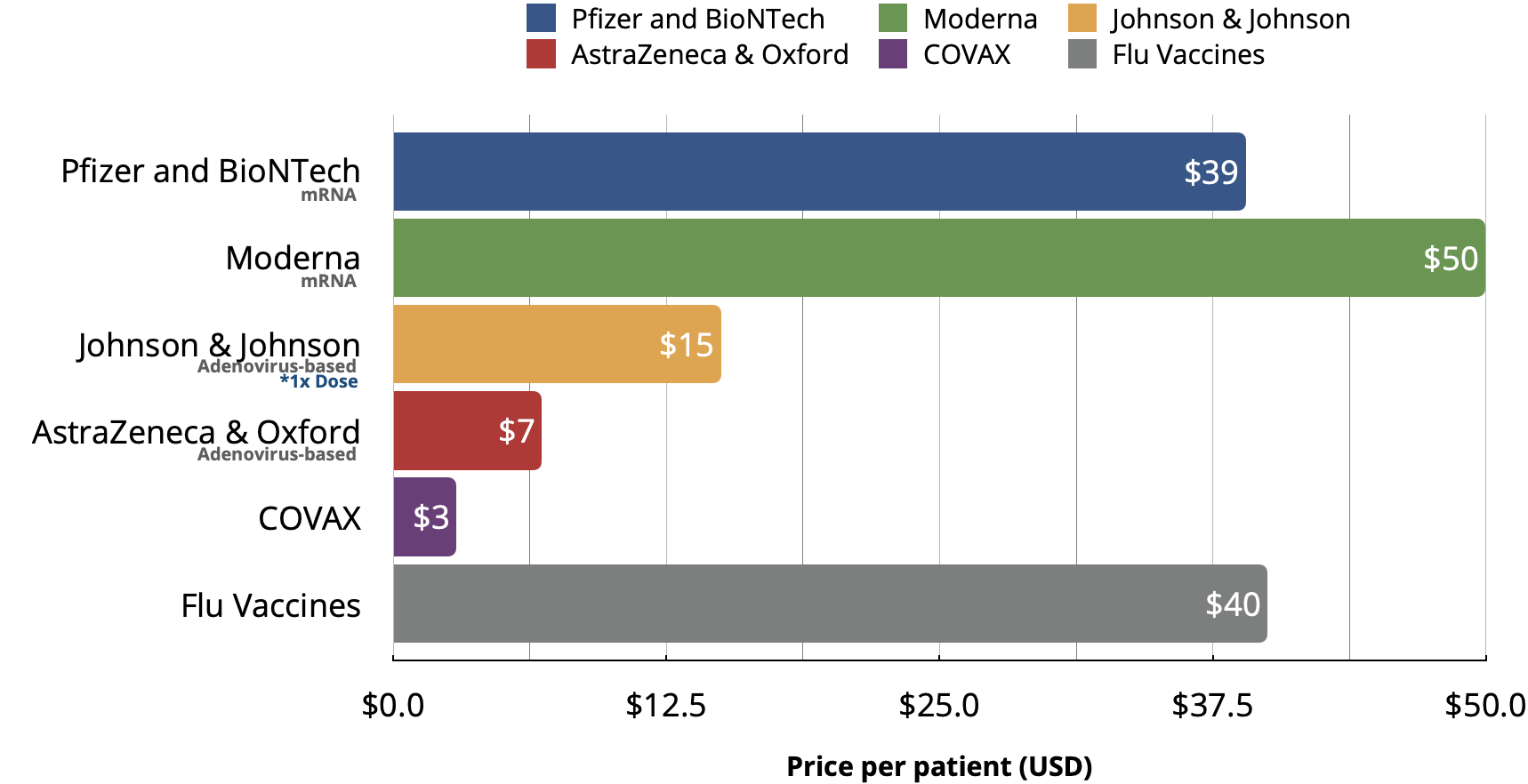
- U.S. Vaccine wholesale prices have been reported to range from 10 to $50 dollars per patient as shown in Fig. 1.
| Vaccine | Type | Doses | Reported Efficacy | Cold Supply Storage | Price per Patient |
|---|---|---|---|---|---|
| Pfizer and BioNTech | mRNA | 2x – 21 days apart | 95% | -75°C | $39.0 |
| Moderna | mRNA | 2x – 28 days apart | 94% | -20°C | $50.0 |
| Johnson & Johnson | Adenovirus-based | 1x | 72% | 7°C | $15.0 |
Pfizer
- Expected to deliver a total of 320 million doses to the U.S. Government:
- 120 million by end of March 2021
- 100 million by the end of May 2021
- 100 million by end of 2021
- Pfizer’s U.S. vaccine production is vertically integrated
- The most critical manufacturing step of its supply chain is combining mRNA production with lipids which occurs during the first half of the manufacturing process
- Pfizer is producing DNA for the U.S. Market with plants located in Kalamazoo, Michigan, St. Louis, Missouri and Andover, Massachusetts
- Critical infrastructures updates during February, 2021 caused a ~40% drop in weekly supply for that month but has since recovered
Moderna
- Expected to deliver a total of 300 million doses to the U.S. Government:
- 100 million by the end of March 2021
- 100 million by the end of May 2021
- 100 million by end of July 2021
- The most critical manufacturing step of its supply chain is the combining mRNA production with lipids which occurs during the first half
- Moderna and Lonza are producing DNA for the U.S. Market with plants located in Norwood, Massachusetts and Portsmouth, New Hampshire respectively
- Moderna is subcontracting several U.S. Pharmaceutical manufacturers to handle drug production and filling
- Lonza
- Corden Pharma
- Catelent Pharma
J&J Janssen
- Expected to deliver a total of 200 million doses to the U.S. Government:
- 20 million by the end of March 2021
- 80 million by the end of June 2021
- 100 million by the end of December 2021
- JJ’s manufacturing cycle is the longest raging from 60 to 80 days
- The most critical manufacturing step of its supply chain is the cell culture growth which occurs at the beginning of the manufacturing process
- Emergent Bio Solutions and Merck Pharmaceutical are producing DNA for the U.S. Market with plants located in Baltimore, MD and Philadelphia, PA respectively
- J&J is collaborating with 5 U.S. Pharmaceutical manufacturers to handle drug production and filling
- Emergent Bio Solutions
- Merck Pharmaceutical
- Grand River Aseptic Manufactoring (GRAM)
- Catalent, Inc
- PCI Pharma Services
U.S. Vaccination Overview and Supply Chain
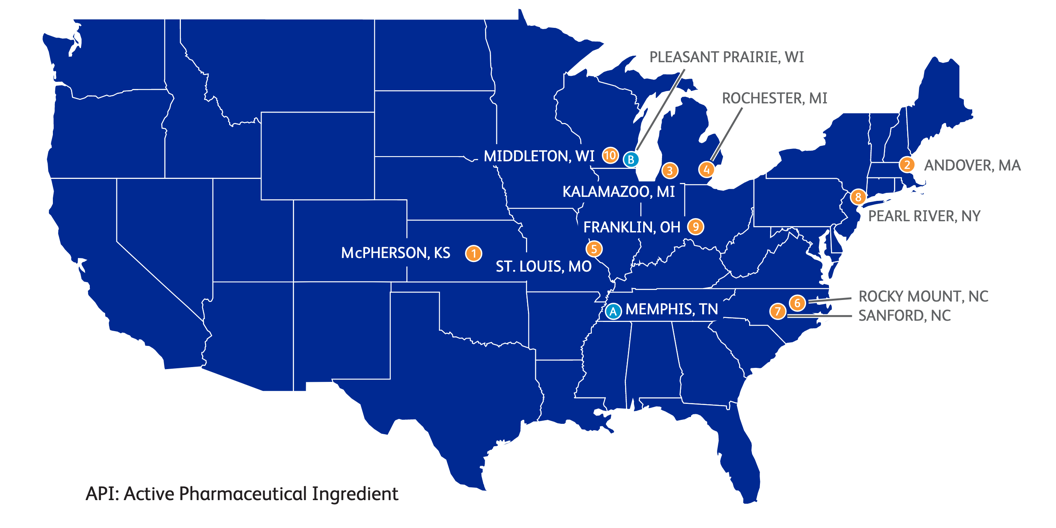
The most important manufacturing, and distribution sites involved in the COVID-19 supply chain in the U.S. are shown in Fig. 2.
Figure 2: Top U.S. COVID-19 Vaccine Supply Chain Suppliers Facilities.
Pfizer and BioNTech
Pfizer can integrate most of its manufacturing and distribution in the United States. The company, however, was part of operation warp speed for distribution (Slaoui & Hepburn, 2020). According to the company’s yearly review, the company has ten manufacturing facilities and two distribution centers, as shown in Fig. 3 . (Inc., 2018), However, only a handful of sites are directly involved in manufacturing its Pfizer-BioNTech COVID-19 Vaccine, as highlighted in Fig. 4 and Fig. 5.

- St. Louis, Missouri plant
- The process starts at this manufacturing plant in the DNA production and raw materials of their COVID-19 Vaccine.
- Drug Formulation in Andover, Massachusetts or Germany facility
- RNA messenger through DNA incubation with genetic building blocks takes place in the Andover facility for the U.S. market while international market is supplied by facilities located in Germany
- Fast shipment to next facility in helicopters or jets at the beggining of the manufacturing process
- Lipids production & other raw materials
- U.K. Croda International
- Formulation, Fill and Finish in Kalamazoo, Michigan
- LNP production step in Kalamazoo is reported to be the bottleneck of the U.S. supply chain. “Combining mRNA and lipids into lipid nanoparticles is the biggest hurdle in the manufacturing process” (Lowe, 2021)
- Two production lines producing 600 vials/min
Moderna
Moderna is a U.S.-based pharmaceutical company founded in 2010 with primary headquarters located in Cambridge, MA, and specialized in the research and development of mRNA-based therapeutics. The company is part of Operation Warp Speed and backed by Venture Capital investments (Moderna, 2021); other takeaways about the company are shown in Fig. 6. The company’s workforce has grown 80% over the past two years, according to LinkedIn data (LinkedIn, 2021).

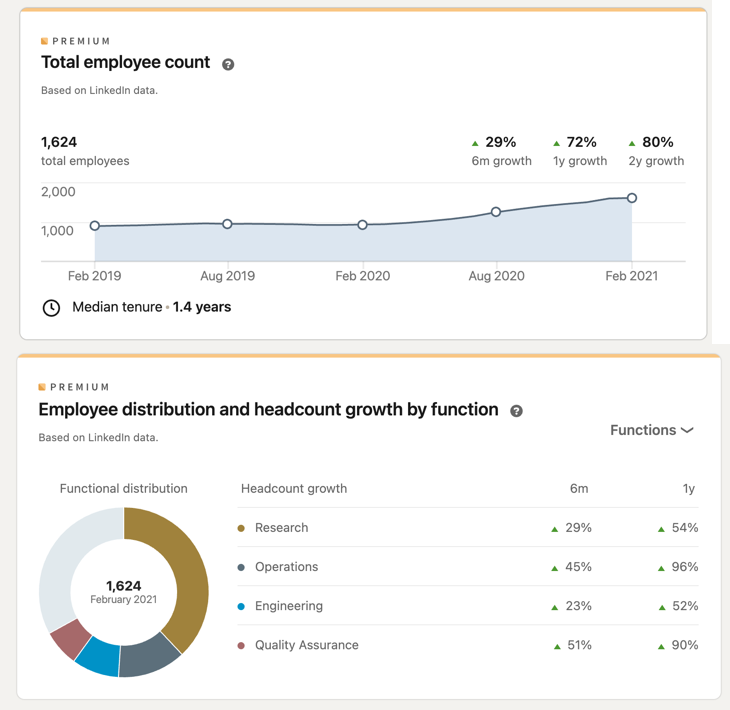
Moderna’s Manufacturing Facilities and Supply Chain Overview
Catalent Pharma is Moderna’s main subcontractor for COVID-19 Vaccine manufacturing in the U.S. (Moderna, 2021),(Neubert, 2021). All U.S. supply comes from Moderna’s dedicated supply chain in the U.S. Supply to locations outside of the U.S. comes from dedicated supply points based outside of the U.S.” (Moderna, 2021)
- Moderna’s Main Plant in Massachusetts
- This plant is involved in the mRNA production phase of the COVID-19 vaccine (Carolyn, 2020)
- DNA Production in Portsmouth, New Hampshire
- Moderna is subcontracting Lonza to produce DNA for their vaccines, one of the world’s largest contract development and manufacturing companies.
- It is reported that at least 3 other production lines located in Visp, Switzerland with a capacity of 600k/day are supplying the international market
- Lipid’s production in Boulder, Colorado
- Moderna is subcontracting Corden Pharma, the largest U.S. pharmaceutical manufacturer to produce enough lipids for its vaccine
- At least 2 production lines were installed in Liestal, Switzerland and Chenôve, France for international market
- Formulation & Fill finishing
- Moderna is utilizing the vast manufacturing network of Catelent formulation and fill finishing.
- Packaging and Distribution
- Moderna is part of the U.S. Operation warp speed for both development and distribution. The company uses the central distribution facility
Moderna’s Cold-Chain Distribution and packaging
- 10 doses of 0.5 each into tray
- 12 trays per package
- Up to 192 packages can be shipped per pallet for a total of 230,400 doses
- Moderna uses UPS, FEDEX, DHL and commercial airlines
- Part of OWS for development and distribution
Moderna Distribution Efforts as of February 23, 2021
•Company has supplied 45.4 million doses of Moderna COVID-19 Vaccine to U.S. Government
•CDC Reports that approximately 30.7 million doses of the Moderna Vaccine has been administered
•Additional 33.2 million doses have been produced and are filled in vials and in the final stages of final production and testing
•Expected to deliver 100 million doses to the U.S. Government by end of March 2021
•Expected to deliver 100 million additional doses by end of May 2021 (moved one month forward) followed by another 100 million additional doses by end of July 2021
Figure 8: Main Moderna’s COVID-19 Vaccine U.S. Manufacturing and Distributions locations.
Johnson and Johnson Janssen COVID-19 Vaccine
Production & Shipments
•March 2021 – 20 million doses
•April to June 2021 – at least 20 million doses per month
J&J’s Janssen Vaccine Distribution Efforts as of March 12, 2021
- FDA Emergency Use Authorization Granted – Feb. 27, 2021
- Company has supplied 2.9 million doses of J&J’s Janssen COVID-19 Vaccine to the U.S. Government
- Plans to distribute 16 million of doses more by the end of March (Services, 2021)
- Johnson & Johnson hopes to have partnerships with a total of 10 manufacturing plants by the end of 2021 (House, 2021)
- Merck will use two of its facilities to produce drug substance, formulate and fill vials (Services, 2021)
- Expected to deliver 100 million doses to the U.S. Government by end of June 2021(House, 2021)
- Expected to deliver 100 million additional doses by end of 2021
J&J is using a global network of collaborators to scale up production of its COVID-19 vaccine as seen in Fig. 9, and highlighted in Fig. 10 and Fig. 11.
Production & Shipments
- March 2021 – 20 million doses
- April to June 2021 – at least 20 million doses per month
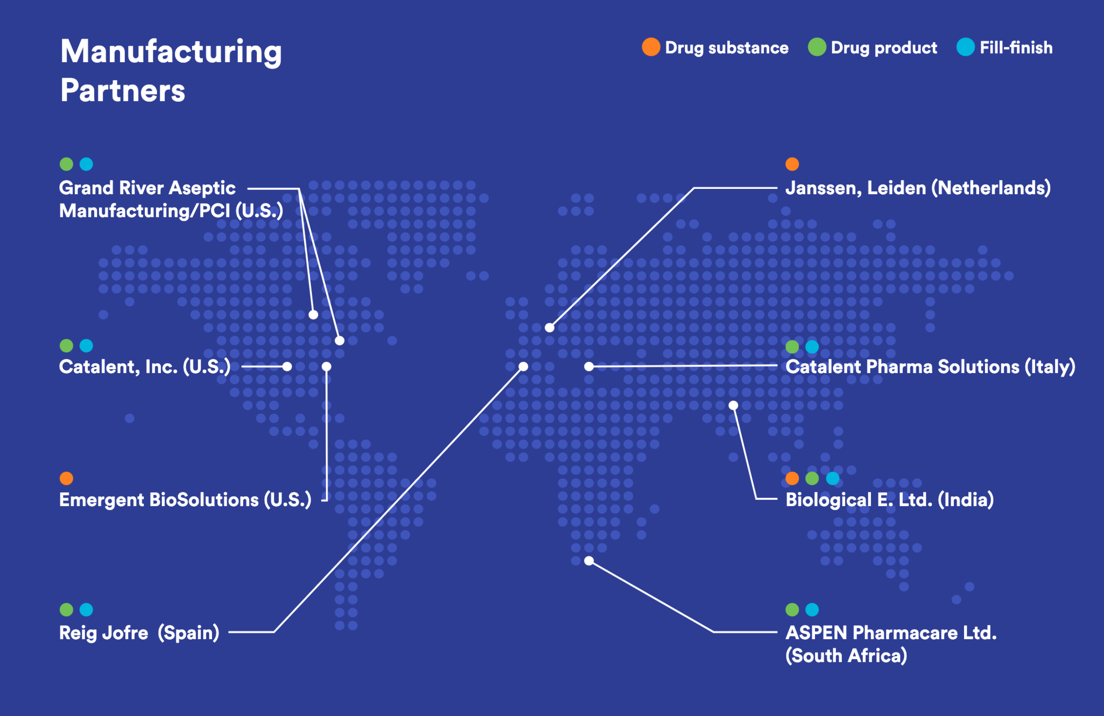
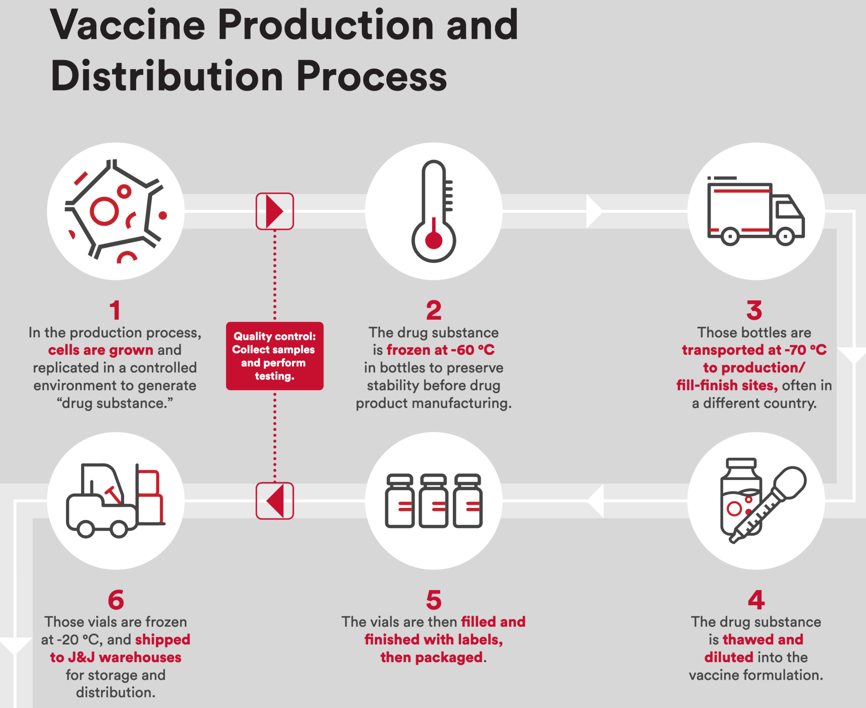
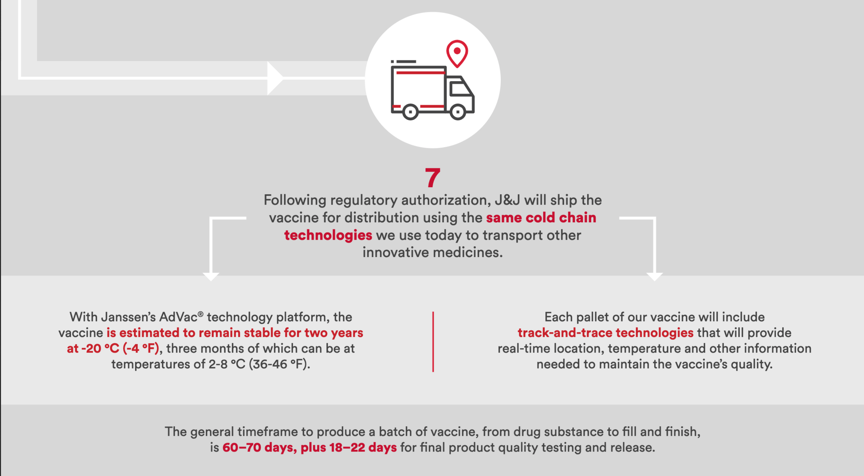
Figure 12: U.S. J&J’s Janssen COVID-19 Vaccine Supply Chain Suppliers Facilities.
J&J Supply Chain Overview
- Drug Substance – Linear DNA Molecules from cells are grown in a bioreactor
- Drug Product – Purification, Fill finishing, and packing
- Distribution McKesson’s OWS Distribution plant
- Cold-Chain Distribution
The vaccine can be frozen at -4° F for up to two years, and once it arrives at its final destination, can stay in a refrigerator at between 35 and 46° F for at least three months.
Each 0.5 mL dose of the Janssen COVID-19 Vaccine is formulated to contain 50 billion virus particles of the Ad26 vector encoding the S glycoprotein of SARS-CoV-2.
COVID-19 Vaccination Landscape
The World Health Organization (WHO) provides weekly bulletins with the status of COVID-19 Vaccine clinical trials in the world. The bulletin is updated twice per week and includes information from several data sources such as Pubmed (Canese & Weis, 2013), the Cochrane vaccine mapping tool (Boutron et al., 2020), ClinicalTrials.gov (Medicine, 2021), WHO International Clinical Trials Registry Platform (ICTRP) (Organization, 2021) and other researchers in industries with registered trials for clinical information (R&D Blue Print, 2021). In addition, several news organizations and research teams have been reporting periodic updates and keeping track of changes, for instance, the New York Times (Carl Zimmer, 2021) and Bloomberg (Bloomberg, 2021), to mention some examples of such efforts to inform the general public.
According to the sources mentioned before, there are 73 vaccines being researched around the world in clinical trials on humans, 16 have reached the final stages of testing, known as Phase III. Of these, at least 182 pre-clinical vaccines are under active investigation in animals as of Feb. 23, 2021
Table 1 tbl. 2 highlights the leading vaccine manufacturers working in their clinical trials and Table tbl. 3 their corresponding vaccine administration medium.
| Number of doses | Schedule | Candidate vaccines | Percentage |
|---|---|---|---|
| 1 dose | 12 | 16% | |
| Day 0 | 12 | ||
| 2 doses | 45 | 62% | |
| Day 0 + 14 | 6 | ||
| Day 0 + 21 | 17 | ||
| Day 0 + 28 | 22 | ||
| 3 doses | 1 | 1% | |
| Day 0 + 28 + 56 | 1 | ||
| TBD / No Data (ND) | 15 | 21% | |
| Total | 73 | 100% |
| Route of Administration | Candidate Vaccine | Percentage | |
|---|---|---|---|
| Oral | 2 | 3% | |
| Injectable | 61 | 84% | |
| SC | Sub cutaneous | 2 | 3% |
| ID | Intra dermal | 3 | 4% |
| IM | Intra muscular | 56 | 77% |
| TBD /No Data (ND) | 10 | 14% |
References
Carolyn, T. W. P. Inc., Y. Johnson. (2020). A vial, a vaccine and hopes for slowing a pandemic — how a shot comes to be. https://www.washingtonpost.com/health/2020/11/17/coronavirus-vaccine-manufacturing/
LinkedIn. (2021). LinkedIn company profile, premium insights. https://www.linkedin.com/company/pfizer/insights/
Lowe, D. (2021). Myths of vaccine manufacturing. https://blogs.sciencemag.org/pipeline/archives/2021/02/02/myths-of-vaccine-manufacturing
Moderna. (2021). Moderna provides u.s. COVID-19 vaccine supply update. https://investors.modernatx.com/news-releases/news-release-details/moderna-provides-us-covid-19-vaccine-supply-update-0#:~:text=16%2C%202021%2D%2D%20Moderna%2C%20Inc,the%20U.S.%20Government%20to%20date.
Neubert, J. (2021). Exploring the supply chain of the pfizer/BioNTech and moderna COVID-19 vaccines. https://blog.jonasneubert.com/2021/01/10/exploring-the-supply-chain-of-the-pfizer-biontech-and-moderna-covid-19-vaccines/




